The U.S. Food and Drug Administration (FDA) has made a historic decision by granting approval to the first-ever medication specifically designed to treat obstructive sleep apnea (OSA), a disorder that affects millions of people worldwide. This approval marks a significant breakthrough in the medical field and offers hope to many individuals suffering from this condition, which has long been treated primarily with devices like Continuous Positive Airway Pressure (CPAP) machines. This newly approved medication could revolutionize the way OSA is managed and offer a more accessible and effective alternative to current treatments.
Understanding Obstructive Sleep Apnea
Obstructive sleep apnea is a serious sleep disorder that occurs when the muscles in the throat relax excessively during sleep, causing a temporary blockage of the upper airway. This results in interrupted breathing patterns throughout the night, leading to reduced oxygen levels in the blood and frequent awakenings. People with OSA often experience symptoms such as loud snoring, choking or gasping for air during sleep, excessive daytime fatigue, and difficulty concentrating. In severe cases, OSA can contribute to a host of complications, including high blood pressure, heart disease, stroke, diabetes, and impaired cognitive function.
The Prevalence of Obstructive Sleep Apnea
It is estimated that approximately 22 million Americans suffer from sleep apnea, with the vast majority being undiagnosed. OSA tends to be more common in individuals who are overweight or obese, but it can affect people of all body types, ages, and genders. The disorder is also more prevalent among older adults and men, although women are increasingly being diagnosed with OSA, particularly after menopause.
The impact of OSA is not limited to physical health; the disorder can significantly affect a person’s quality of life. The constant fatigue and sleep deprivation associated with OSA can lead to difficulties in the workplace, strained relationships, and a decreased ability to engage in everyday activities. Furthermore, untreated OSA can contribute to long-term health complications, making early diagnosis and treatment crucial.
Traditional Treatments for Obstructive Sleep Apnea
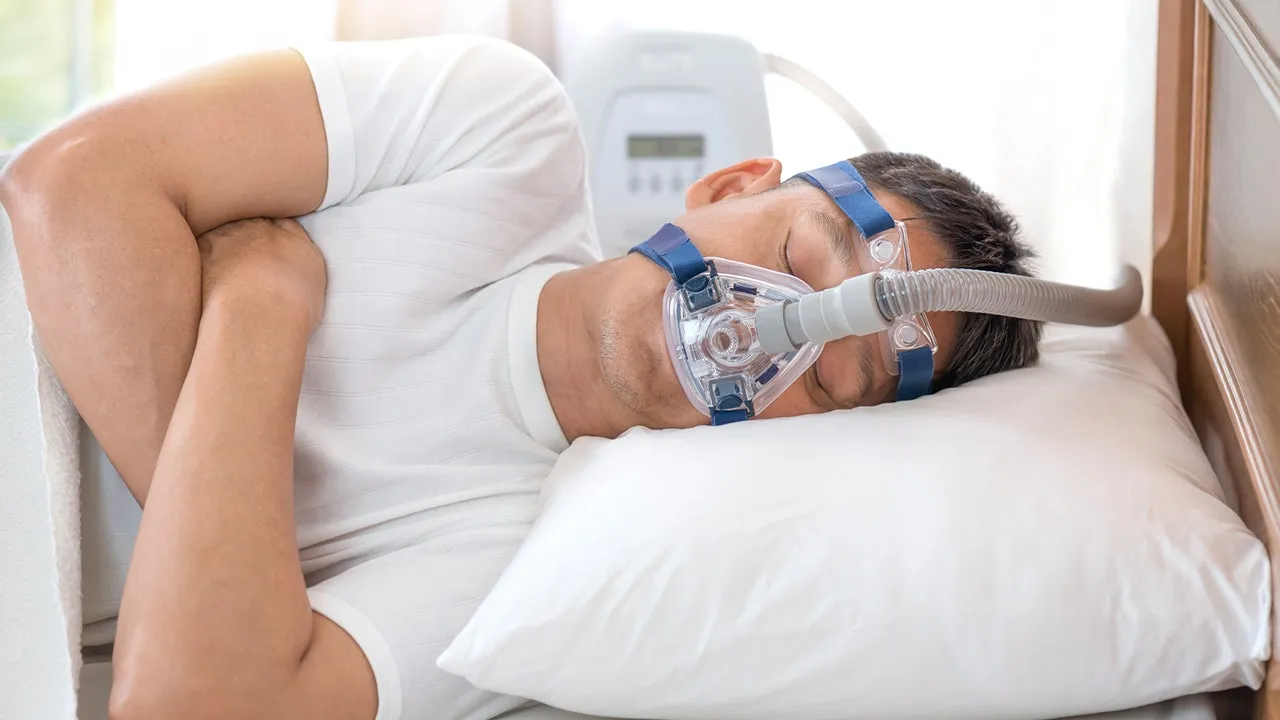
For many years, the standard treatment for OSA has been the use of CPAP machines. CPAP, or Continuous Positive Airway Pressure, is a device that delivers a constant flow of air through a mask to keep the airway open during sleep. While highly effective in treating the disorder, CPAP therapy is often met with resistance from patients due to the discomfort of wearing a mask and the noise generated by the machine. As a result, adherence to CPAP therapy is a common challenge, with many patients abandoning treatment after a short period.
In addition to CPAP, other treatment options for OSA include lifestyle changes, such as weight loss, smoking cessation, and the reduction of alcohol intake, as these factors can exacerbate the severity of the condition. Oral appliances, which are worn in the mouth to reposition the jaw and keep the airway open, are another alternative for some individuals, particularly those with mild to moderate OSA.
For severe cases of OSA, surgical options may be considered, including procedures to remove excess tissue from the throat or reposition the jaw. However, surgery is typically reserved for patients who do not respond to other treatments.
Despite these options, many people with OSA continue to struggle with managing their condition. This has led to a growing demand for new treatments that are more convenient, effective, and accessible.
The FDA Approval of the First Medication for OSA
In response to this need, the FDA has approved the first-ever medication specifically designed to treat obstructive sleep apnea. This groundbreaking approval comes after years of research and clinical trials, and it offers a new hope for individuals who have struggled with traditional treatments or have been unable to find an effective solution for their sleep apnea symptoms.
The Medication: A New Approach to OSA Treatment
The newly approved medication, a novel oral drug, works by targeting the underlying causes of OSA. Unlike CPAP therapy, which addresses the mechanical obstruction of the airway, this medication acts on the central nervous system to stimulate the muscles of the upper airway, preventing collapse during sleep. By keeping the airway open without the need for a mask or machine, this medication provides a more convenient and comfortable option for patients who may have difficulty using traditional therapies.
In clinical trials, the medication has shown promising results in reducing the frequency and severity of apneas, as well as improving overall sleep quality. Patients who took the medication experienced fewer interruptions in their breathing patterns, less daytime sleepiness, and improved cognitive function. Furthermore, the medication was well-tolerated, with minimal side effects reported.
The approval of this medication represents a major step forward in the treatment of obstructive sleep apnea, as it addresses a key challenge for many patients: adherence to therapy. By offering a non-invasive and easy-to-use alternative, the medication has the potential to significantly improve patient outcomes and quality of life.
How the Medication Works
The new medication for OSA operates by influencing the brain’s respiratory control centers. It stimulates the muscles around the airway to prevent them from collapsing during sleep, which is a primary cause of obstructive sleep apnea. The mechanism of action is different from CPAP therapy, which applies continuous pressure to the airway to keep it open mechanically. Instead, the medication offers a pharmacological solution that directly targets the physiological aspects of the disorder.
In essence, the medication works by increasing muscle tone in the upper airway during sleep, allowing the airway to remain open and reducing the frequency of apneas and hypopneas (partial blockages of the airway). This improvement in airway patency results in fewer interruptions in breathing, allowing individuals to experience deeper and more restful sleep.
Dosage and Administration
The medication is taken orally, typically once a day before bedtime. It is designed to be taken on a long-term basis, with patients instructed to continue taking it consistently in order to experience the full benefits. The exact dosage and administration instructions will be determined by a healthcare provider, based on the patient’s specific needs and medical history.
The medication is expected to be well-tolerated, with minimal side effects. However, as with any medication, there may be some risks, and patients are advised to consult with their healthcare provider before starting treatment. It is also important for patients to continue monitoring their condition and work with their doctor to ensure that the medication is providing optimal results.
The Impact of FDA Approval
The approval of the first medication for OSA has the potential to reshape the landscape of sleep medicine. For many years, the only viable treatment options for obstructive sleep apnea were CPAP therapy and surgical interventions. While CPAP is effective, its low adherence rates have made it an imperfect solution for many patients. The new medication offers a viable alternative, especially for those who struggle with the discomfort and inconvenience of CPAP machines.
Accessibility and Convenience
One of the primary advantages of this medication is its accessibility and convenience. Unlike CPAP therapy, which requires specialized equipment and can be cumbersome to use, the medication can be taken at home without the need for any devices. This makes it a more appealing option for individuals who may have difficulty incorporating CPAP into their daily routine.
Furthermore, the medication may be especially beneficial for patients who have mild to moderate OSA, as it offers a non-invasive and less intrusive treatment option. For those who have been hesitant to try CPAP therapy or have experienced poor results with other treatments, this medication could offer a much-needed solution.
A New Era for Sleep Medicine
The approval of this medication represents a new era in the treatment of obstructive sleep apnea. It opens the door for further research and innovation in the field of sleep medicine, paving the way for additional pharmacological treatments that could address other aspects of OSA or related sleep disorders. This breakthrough could ultimately lead to a broader range of treatment options, allowing healthcare providers to tailor their recommendations to individual patients’ needs.
Moreover, the availability of this medication could help reduce the stigma associated with sleep apnea treatment. Many individuals with OSA feel self-conscious about using CPAP machines, particularly in social situations. The approval of an oral medication provides a discreet and private alternative, which may encourage more people to seek treatment and take control of their health.
Implications for Public Health
The approval of this medication has significant implications for public health. With millions of people worldwide affected by obstructive sleep apnea, the new medication has the potential to improve the lives of countless individuals. By offering a more effective and convenient treatment option, the medication could reduce the prevalence of untreated OSA, which is associated with a range of serious health complications, including cardiovascular disease, diabetes, and stroke.
Additionally, improving adherence to OSA treatment could help mitigate the economic burden of the disorder. Untreated sleep apnea is linked to higher healthcare costs due to increased hospitalizations, doctor visits, and the management of related health conditions. By providing a more accessible and effective treatment option, this new medication could help reduce the long-term healthcare costs associated with OSA.
Ongoing Research and Future Developments
The approval of the first medication for OSA is just the beginning of what promises to be an exciting and transformative period in the field of sleep medicine. Ongoing research is focused on refining and improving this treatment, as well as exploring new medications and therapies that could further enhance the management of OSA and related sleep disorders.
As the understanding of sleep apnea continues to evolve, scientists and healthcare providers are working to develop more personalized treatment approaches that address the specific needs of each patient. This may involve a combination of pharmacological treatments, lifestyle modifications, and other interventions that work together to improve both the quality of sleep and overall health outcomes.
With the approval of this groundbreaking medication, the future of sleep apnea treatment looks brighter than ever before, offering hope to millions of individuals who have long struggled to find effective solutions for their condition.