First Lyme Disease Vaccine in 20 Years Begins Clinical Trial
Pfizer and Valneva recently disclosed that a new vaccine aimed at preventing Lyme disease is advancing to the last stage of clinical trials. If approved, this vaccine could provide Americans with an additional safeguard against the tickborne disease, which may lead to as many as 476,000 illnesses each year.
The vaccine candidate ( VLA15 ) is currently being tested in humans with a regimen consisting of four doses – three primary doses and one booster dose. With promising results thus far, researchers are gearing up for the Phase 3 clinical trial to assess the vaccine’s efficacy and safety.
With the growing incidence of Lyme disease worldwide, the researchers emphasize the importance of offering a fresh option to protect people from this ailment. They hope that the Phase 3 study findings will further substantiate the favorable evidence supporting VLA15.
Below are the details we have gathered on the new vaccine, its function in preventing Lyme disease, and the projected timeframe for its distribution.
The Vaccine Solution for Lyme disease.
In the U.S., Lyme disease, which is primarily caused by Borrelia burgdorferi bacteria, stands as the most prevalent vector-borne disease. The transmission occurs through blacklegged ticks infected with the bacteria, and if you live in an endemic region, you are likely aware of this disease.
According to Bobbi Pritt, MD, chair of the division of clinical microbiology at Mayo Clinic, Lyme disease has become a well-known issue that is causing alarm and apprehension among the public. She emphasized that the current scientific capabilities make it feasible to produce a vaccine for Lyme disease.
Once approved, VLA15 will stand as the exclusive Lyme disease vaccine in the U.S., despite not being the first vaccine employed for prevention. LYMERix, an earlier vaccine, was utilized for a brief period in the early 2000s and exhibited an impressive 80% reduction in infections. However, due to numerous complaints of arthritis and other adverse effects, demand for LYMERix drastically declined, ultimately leading to its discontinuation by the FDA in 2002.
In the last two decades, there has been a growing necessity for a vaccine. The changing climate has led to a higher likelihood of human-tick interactions due to earlier spring seasons and prolonged summers. The expanding populations of mice and deer, which serve as hosts for ticks, coupled with increased outdoor activities in new locations, may also be responsible for the rise in cases and risks.
VLA15, the latest multivalent protein subunit vaccine, is designed to target the outer surface protein A (OspA) of Borrelia burgdorferi. OspA is a protein that is abundantly present on the bacteria, and if the body has previously encountered it through vaccination, a robust immune response can aid in combating the bacterium as soon as it possibly even enters the body.
The vaccine’s ability to target various proteins on different bacteria strains gives it a multivalent characteristic, resulting in broader coverage.
The Pfizer spokesperson mentioned that the potential vaccine is set to improve upon earlier versions by covering OspA serotype 1 found in North America, along with serotypes 2 through 6 to target the main Borrelia strains causing Lyme disease in Europe.
The upcoming Phase 3 trial will enroll around 6,000 participants aged 5 and above from 50 different sites where Lyme disease is prevalent. These sites include countries like the U.S., Finland, Germany, the Netherlands, Poland, and Sweden. Participants will be given either three doses of the vaccine or placebo as a primary series, followed by a single booster dose of the vaccine or placebo.
When Will the Lyme disease Vaccine Be Available?
While the VLA15 vaccine shows promise in safeguarding those vulnerable to Lyme disease in the U.S. and Europe, it could be a few years before it becomes widely available for administration.
There is no set schedule for when information about VLA15 will be made public, but according to the Pfizer representative, the research will take nearly two and a half years. With this timeframe in mind, it might not be until 2025 that we find out if the vaccine could be widely accessible after potential FDA approval.
Our objective is to provide a secure and efficient vaccine that safeguards individuals who may encounter infected ticks, potentially addressing this increasing unmet requirement. Considering the public’s lack of confidence in the previous Lyme disease vaccine, the clinical trial will diligently monitor for any potential side effects or risks. Dr. Pritt emphasized

In the future, if the vaccine proves to be both safe and effective, Dr. Pritt envisions a scenario where the VLA15 shot is readily accessible to individuals who desire it, particularly those who frequent outdoor areas in the Upper Midwest and Upper Northeast regions, as well as certain parts of Europe where the disease is prevalent.
Dr. Pritt mentioned that the vaccine would be accessible to at-risk populations, but it wouldn’t be advised for everyone. Individuals who spend a significant amount of time outdoors and are potentially exposed to ticks, such as hiking and camping enthusiasts or park rangers, would greatly benefit from receiving the vaccine.
Preventive Measures for Lyme Disease
The most effective ways to prevent Lyme disease until a vaccine is introduced are to avoid wooded areas, use bug spray that is approved by the EPA, and thoroughly inspect your body and clothing for ticks after spending time outside.
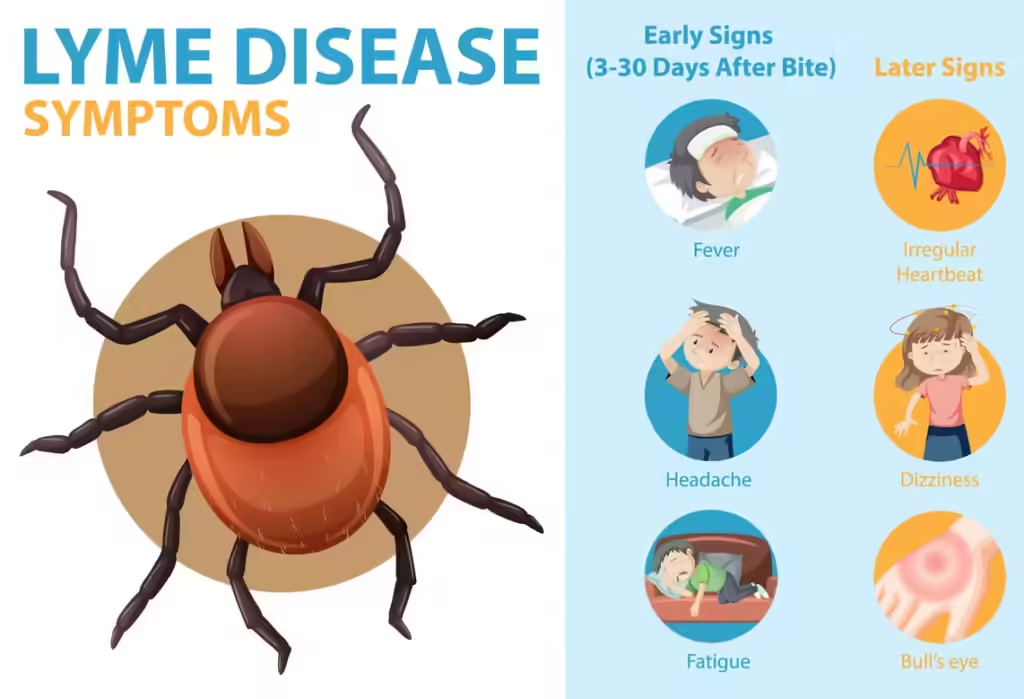
According to Dr. Pritt, a significant number of Lyme disease cases go unnoticed due to lack of tick bite recollection. Recognizing symptoms early is essential, as timely treatment with antibiotics can lead to better outcomes.
Despite the potential game-changing effects of VLA15 in protecting against Lyme disease, it’s crucial to remember the tried-and-true basic prevention strategies that are consistently effective.
Dr. Pritt reiterated the significance of tick bite prevention, recommending the use of bug spray even with vaccination. While vaccines are beneficial, they are not 100% effective, so additional measures should be taken to protect oneself outdoors.




